Pharmaceutical companies ideally look for opportunities to strength their services offering by choosing the merge with small and large pharma and biotech firms. CDMO provides critical services such as vital drug research, specialised drug development, and final medication manufacture. The use of contract development and manufacturing services not only use in pharmaceutical services but also reduces their operational cost. Contract Development and Production Organizations (CDMOs) are sectors of the pharmaceutical business that support pharmaceutical corporations with medication manufacturing, research, and development.
Market Dynamics – Asia Pacific Pharmaceutical CDMO Market
The Pharmaceutical CDMO market is expected to grow due to some of the factor which is responsible for its growth. Due to the increasing demand of generic drug in these region the market is expected to grow during the forecast period. Cost benefits owing to low labour cost and capital expenditure are the key factors for outsourcing the Asia Pacific region. Besides, in Asia Pacific, India is one of the country established as prominent player in the manufacturing of solid dosage form for the large scale production of generics drugs. The source of APIs has such a large impact on their profitability, generic pharmaceutical companies are more inclined than innovator pharmaceutical companies to cooperate with Asian CMOs. The cost of raw materials is slightly less than half of the total cost of sold goods for generic producers. In such a competitive environment, Asian CMOs' ability to offer a low pricing is important.
Economic Impact of COVID-19 on Asia Pacific Pharmaceutical CDMO Market
The exclusive COVID 19 impact analysis report by Axiom MRC provides a 360 degree analysis of micro and macro-economic factors on Asia pacific Pharmaceutical CDMO market. Also, complete analysis of changes on healthcare expenditure, economic and international policies on supply and demand side. The report also studies the impact of the pandemic on global economies, international trade, business investments, GDP, and marketing strategies of key players present in the market. The COVID-19 outbreak is having a substantial impact on the Pharmaceutical CDMO industry. Strict laws and regulations due to covid-19 put an impact on end-use markets, supply chains are disturbed, and the competitive order of producers and manufacturers.
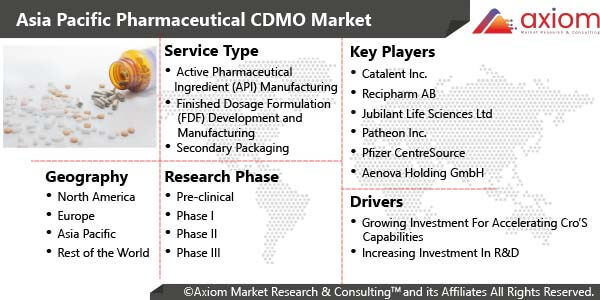
Asia Pacific Pharmaceutical CDMO Market - Segmental Overview
The study analysed the Asia pacific pharmaceutical CDMO market based on service type, research phase and geography
Asia Pacific Pharmaceutical CDMO Market by Service Type
Based on service type, the market is segmented into the active pharmaceutical ingredient (api) manufacturing, finished dosage formulation (FDF) development and manufacturing, secondary packaging. Finished Dosage Formulation (FDF) is sub segmented into the solid dose formulation, liquid dose formulation and injectable dose formulation. Based on services types the active pharmaceutical ingredient (API) manufacturing segment is dominate the Asia Pacific Pharmaceutical CDMO Market due to adoption of outsourcing by pharmaceutical companies and focusing on drug development process.
Asia Pacific Pharmaceutical CDMO Market by Research phase
Based on the Research Phase the market is segmented into the Pre-clinical, Clinical like Phase I, Phase II, Phase III and Phase IV. The Pre-clinical followed by Phase I, among others are likely to witness increasing demand for market over the forecast period due to increasing clinical research for newly invented drugs in the research.
Asia Pacific Pharmaceutical CDMO Market by Country
By country, the pharmaceutical CDMO market is studied across the countries of China, India, Japan, and rest of APAC. China is projected to have considerable share in the Asia Pacific Pharmaceutical CDMO market due to significant investment in the drug discovery and development in the country. The increasing drug discovery and development in this region is expected to grow during the forecast period.
Asia Pacific Pharmaceutical CDMO Market Key Players
The key competitor of the market includes, Catalent Inc., Famar S.A., Hospira, Inc., Jub AbbVie Inc., Almac Group Ltd., B. Braun Melsungen AG, Boehringer Ingelheim, International GmbH, Lonza Group Ltd., Pfizer Inc. ilant Life Sciences Ltd., Lonza Group, Patheon Inc., Pfizer CentreSource, Recipharm AB.